A lovely example of a neutralisation reaction that will wow all but the most hardened 10-year-olds!
This chemistry demonstration illustrates the effervescent reaction between acid, base, and universal indicator producing a beautiful 'rainbow' effect.
Perfect for an open evening, this engaging science experiment will spark excitement and enthusiasm in year 6 pupils and their parents.
"Rainbow Fizz" is simple to set up and run during your school's open evening and will be a memorable introduction to the world of secondary school science and a fantastic way to foster interest in STEM subjects.
Incorporating engaging science experiments like our "Rainbow Fizz" into your school's open evening is an excellent way to ignite curiosity and excitement in Year 6 students as they prepare for their journey to secondary school.
By participating in this experiment, students get a taste of the scientific method and the excitement of discovery. It's a memorable introduction to the world of secondary school science and a fantastic way to foster their interest in STEM subjects.
 Equipment:
Equipment:
- Goggles
- Test Tubes
- Stand, boss and clamp
- Pipettes
- Universal indicator and colour chart
- Hydrochloric acid (HCl) 0.5 moldm-¹ (not considered hazardous at this concentration)

- Sodium carbonate (Na₂CO₃ ) – freshly made


Looking for more science open evening inspiration?
 Method:
Method:
- Make up the sodium carbonate solution, adding 33 g sodium carbonate to 100 mL distilled water and mix to dissolve. This solution is harmful and should be transferred to an appropriately labelled stoppered bottle.
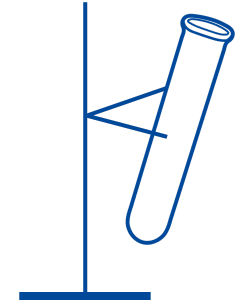
- Set up the clamp stand to hold the test tube at a slight angle.
- Fill the test tube about 2/3 full of hydrochloric acid.
- Add about 3 mL universal indicator the test tube (this will settle near the top of the acid, and will turn the acid red).
- Carefully add about 3 mL sodium carbonate solution to the test tube by allowing it to run down the inside of the tube (use a pipette). The carbonate solution will sink to the bottom and turn purple in colour. The solution will fizz.
- Carefully return the test tube to the upright position and watch as the rainbow develops.

 Notes for your science open day:
Notes for your science open day:
- You can make this more spectacular by setting it up in a burette (make sure the tap is closed).
- If you leave the reaction over time, it should all turn green as the whole solution finishes reacting (and becomes neutralised).
Get in touch
If you have any questions or require additional information, please don't hesitate to get in touch with us.
We're here to support you in creating an unforgettable science open evening that sparks the curiosity and enthusiasm of future scientists!
Why not check out some of our other science open evening demonstration ideas!